One of the most effective spin techniques used by advocates of “integrative medicine” (also sometimes called “complementary and alternative medicine,” or CAM for short) to legitimize quackery has been to claim basically all non-pharmacologic, non-surgical interventions as “integrative,” “complementary,” or “alternative.” Thus, science-based interventions such as diet changes to treat and/or prevent disease, exercise, and other lifestyle alterations are portrayed as somehow so special that they need their own specialty, “integrative medicine,” even though they are simply part of medicine. I pointed this out a mere two weeks ago when I discussed the National Center for Complementary and Integrative Health (NCCIH) review of non-pharmacological treatments for pain. It was a systematic review that was essentially negative but spun as positive for some interventions and lacked some key analyses that a good systematic review includes, such as assessment of the quality of the studies included and evaluating them for bias.
Such were my thoughts over the weekend as I got into a Twitter exchange with an advocate of integrative medicine who was touting the benefits of diet as a cancer preventative and how a course in nutrition “opened her eyes.” That in and of itself wasn’t particularly annoying, although I strongly suspect that the nutrition course she took was not given by actual registered dietitians or other experts in science-based nutrition (she wouldn’t say when questioned). What was annoying is that she trotted out some tropes beloved by integrative medicine proponents, such as the claim that most doctors don’t do prevention because they get paid to treat. She was called out for it:
.@DrKristieLeong "Not all docs emphasize prevention because they get paid to treat it." How else to interpret that? pic.twitter.com/5mMmpxGDKC
— Doc Bastard (@DocBastard) September 17, 2016
Oddly enough, on the same day a post from the American Society of Clinical Oncology (ASCO) came up in e-mail lists that discussed the actual evidence for the utility of diet and exercise for cancer prevention. It’s almost as though Twitter were telling me it was time for me to discuss this issue from a science-based perspective. So I will attempt to do so.
Diet and exercise versus cancer: The evidence
As I was in the middle of this Twitter exchange, which was much longer than most that I indulge in mainly because my wife was away for the day, I saw in my e-mail in box this article, Impact of Adherence to Cancer Prevention Guidelines on Diet, Physical Activity on Cancer Risk and Mortality (PDF). My first thought when reading this summary was that this is cool, something I need to read. My second thought was, given my recent Twitter exchange now would be a good time to blog about it. My third thought, which came as I went searching for the actual study, is why it took ASCO two months to feature the story, given that the actual study by Lindsay N. Kohler, MPH and co-authors had been published in July in Cancer Epidemiology, Biomarkers & Prevention. I also noted that it came from the University of Arizona, which did raise red flags for me given that that is the home of the godfather of “integrative medicine,” Andrew Weil, but fortunately the systematic review came out of the Department of Nutritional Sciences and the Mel and Enid Zuckerman College of Public Health. That’s not to say that I wasn’t concerned somewhat, given that the Zuckerman College of Public Health made a deal last year with Southwest College of Naturopathic Medicine to offer a dual-degree program for a naturopathic medical degree (ND) and a master’s degree in public health (MPH), slated to begin this term.
Still, I decided to take a look at the review on its merits. It begins with a discussion of existing dietary and exercise guidelines for health and cancer prevention developed by the U.S. Department of Health and Human Services along with health organizations such as the American Cancer Society and the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR). These cancer prevention and health promotion guidelines focus on specific lifestyle recommendations to
- Achieve and maintain a healthy weight throughout life
- Adopt a physically active lifestyle
- Consume a healthy diet with an emphasis on plant-based foods
- Limit alcohol consumption
Looking at the detailed ACS guidelines (lay version here) originally published in 2012 and the WCRF/AICR guidelines, I realize that I’m probably not doing so great. Granted, I recently started a concerted effort to alter my diet and lose about 20-25 lbs, which would bring me back to a pretty healthy weight, and to exercise by bicycling several miles five or more times a week, taking more brisk walks, and taking the stairs instead of the elevator more often, but I still am sedentary a lot and really have a hard time sticking to the more plant-based diet. In this, I rather suspect that I’m not unlike a lot of middle-aged guys whose jobs don’t involve physical labor.
My own personal difficulties grappling with trying to adopt a healthier lifestyle aside, Kohler et al sought to determine whether adherence to the ACS guidelines is associated with a decreased risk of cancer. In other words, do the guidelines work? To this end, they undertook a systematic review of the epidemiology literature, focusing only on prospective cohort studies. That means that the investigators followed the subjects in their cohorts only after they were enrolled in the study. Prospective studies are generally considered a higher level of evidence than retrospective studies (studies that look at patients in the past) because recall bias can be decreased, objective measurements can be taken in a standardized fashion, and the effect of confounders can be decreased. Here’s how the studies were chosen:
Only prospective cohort studies were eligible for inclusion, as the focus was to ascertain cancer incidence and cancer mortality. Minimally, studies must have collected data for physical activity and diet, generated an adherence score on the basis of either ACS or WCRF/AICR cancer prevention guidelines (2, 12), and reported cancer outcomes of incidence and/or mortality to be deemed eligible for this review. Overall cancer incidence and cancer mortality were the primary outcomes of interest. However, site-specific cancer risks were also considered when data were available from at least two studies meeting the eligibility criteria. Commentaries and summary documents were excluded unless they presented additional data.
Resulting in the following studies being chosen from the database databases the current recommendations of Preferred Reporting Items for Systematic Reviews and Meta-analysis Approach (PRISMA):
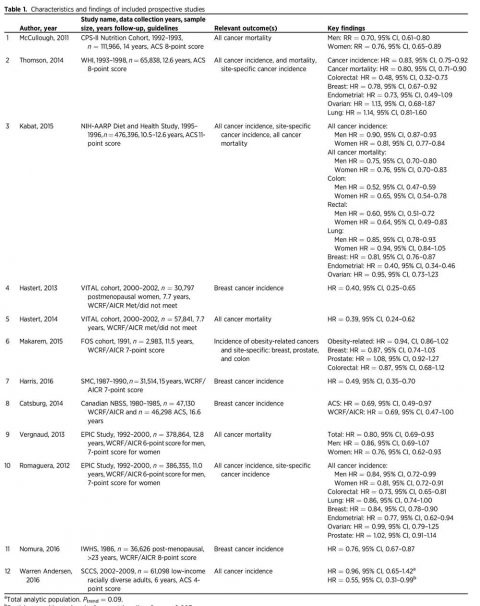
A total of 2,033 potentially relevant studies were reviewed; after removal of duplicates and exclusion on the basis of title or abstract, 25 full articles on nutrition and physical activity cancer prevention guideline adherence were retained for in-depth consideration. The selection process for the articles is shown in Fig. 1. We identified 12 articles that met the a priori criteria for inclusion (Table 1). These studies represented analyses of data from 10 cohorts including the Cancer Prevention Study-II (CPS-II) nutrition cohort (13), the Women’s Health Initiative (WHI) cohort (14), the NIH-American Association of Retired Persons (NIH-AARP) Diet and Health Study cohort (15), the Framingham Offspring (FOS) cohort (16), the Vitamins and Lifestyle (VITAL) Study cohort (17), the Canadian National Breast Screening Study (CNBSS; ref. 18), the Swedish Mammography Cohort (SMC; ref. 19), the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort (20, 21), the Southern Community Cohort Study (SCCS; ref. 22), and the Iowa Women’s Health Study (IWHS) cohort (23).
Reviewing the 12 studies, Kohler et al found that high adherence to established nutrition and exercise guidelines was associated with decreases of 10% to 61% overall cancer incidence and mortality. The study finding the greatest reduction in risk was the analysis of the VITAL study by Hastert and colleagues, which found that meeting at least five recommendations from the WCRF/AICR guidelines compared with meeting none demonstrated a 61% reduction in cancer-specific mortality over 7.7 years of follow-up (HR, 0.39; 95% CI, 0.24–0.62). The study that showed the lowest decrease was a study utilizing the ACS guidelines including nearly a half a million men and women aged 50-71 years in the NIH-AARP Diet and Health Study. Basically, Kabat and colleagues reported a statistically significant decrease in cancer incidence over the 10.5 years of follow-up for both highly adherent men (HR, 0.90; 95% CI, 0.87–0.93) and women (HR, 0.81; 95% CI, 0.77–0.84). A statistically significant reduction in cancer mortality was also reported during the 12.6 years of follow-up for both highly adherent men (HR, 0.75; 95% CI, 0.70–0.80) and women (HR, 0.76; 95% CI, 0.70–0.83; ref. 27). In fact, here’s a table summarizing the studies (click to embiggen, if necessary):
Yes, the figures are all over the place, but there is a consistent association with adherence to the ACS or WCRF/AICR dietary guidelines with decreased overall incidence of and mortality from cancer. The variability can likely be explained by the differences in the cohorts (for instance, the NIH-AARP cohort consisted only of people over 50, an age when people enter the most cancer-prone period in their lives), how adherence scores were defined, and the like. But what about the effect of diet and exercise on individual cancers?
Diet and exercise versus individual cancers
The authors also looked at the effect of adherence to these diet and exercise recommendations on the incidence and risk of mortality from individual cancers, primarily breast, colorectal, lung, and endometrial cancer, because these are the cancers for which the most studies were available.
For breast cancer, there were eight studies reporting results for breast cancer. Kohler et al note that there were consistent reductions in breast cancer incidence reported in the WHI, NIH-AARP, and EPIC cohorts for high adherence to nutrition and physical activity cancer prevention guidelines versus low adherence, for a reduction in risk. Also, meeting at least five WCRF/AICR recommendations compared with meeting none was associated with a 60% reduction in breast cancer incidence. Overall, the reduction in risk associated with high adherence to guidelines compared to low adherence ranged from 19%–60%, although one of the studies did not reach statistical significance.
Results were similar, although there were fewer studies, for colorectal cancer and endometrial cancer. Basically four of these studies reported results for colorectal cancer specifically, with decreased risk of this disease found in the WHI cohort and the NIH-AARP cohort, as well as the EPIC cohort. The FOS cohort showed a trend towards decreased risk of colorectal cancer that did not reach statistical significance. Taken together, the four studies suggested a reduction in risk associated with adherence to diet and exercise guidelines ranging from 27%–52%. In the case of endometrial cancer, there were three studies that examined this cancer as an endpoint. Both the NIH-AARP and EPIC cohorts both found decreased risk associated with higher adherence to guidelines, and, again, there was one cohort (WHI) with a finding of decreased risk that did not reach statistical significance; the overall decrease in risk reported was 23%–60%.
Finally, the authors examined lung cancer. Of course, lung cancer is unique in that it is a cancer for which the main lifestyle cause is unequivocally known: smoking tobacco products. Although Kohler et al characterize the effect of adherence to diet and exercise guidelines on lung cancer risk as “equivocal,” my reading of the studies they report leads me to believe that adherence to the diet and exercise probably has little or no effect on the risk of lung cancer. This is not particularly surprising given how powerful the effect of smoking is on lung cancer risk and how uncommon lung cancer not associated with smoking is.
What did surprise me is that there was no association with prostate cancer incidence noted utilizing the ACS guidelines in the NIH-AARP cohort or the WCRF/AICR guidelines in the EPIC or FOS cohorts given that prostate cancer is one cancer commonly invoked as being preventable by diet. On the other hand, the diets touted for preventing prostate cancer are more extreme than those encompassed by the ACS and WCRF/AICR guidelines because they are usually vegan. The authors also found no reduction in risk associated with high adherence to diet and exercise guidelines versus low adherence for ovarian cancer.
Diet and exercise versus cancer: Putting it all together
Physicians have sought to identify modifiable risk factors for cancer for a very long time now, possibly as far back as Hippocrates. Of course, two such factors that rapidly rise to the top of any list for just about any disease must be diet and exercise, and, contrary to the frequent criticism of “conventional” medicine by those advocating “integrative” approaches, a great deal of research has been done examining the effects of various diets, exercise, and the like on the risk of cancer in general and of specific cancers. Unlike the case of drug therapies and other discrete treatments, it’s a lot harder to tease out the effects of diet and exercise on cancer risk because such studies take a lot of subjects (one of those cohorts has half a million subjects) and, because the most common cancers are generally diseases of aging, a long period of time. Moreover, while it’s possible to do a randomized controlled trial for many interventions, the complexities of diet make studying the effects of nutrition in such a manner very difficult. That’s not to say that it can’t be done. Indeed, it is being done, but the same problem exists: Lots of patients and lots of time are still required, which has led some to question whether RCTs are superior to prospective cohort studies for such interventions. The point, of course, is that physicians and scientists, contrary to the stereotype, have not been “ignoring” the role of diet in diseases like cancer. Quite the contrary.
This particular systematic review is touted as the first to examine dietary and physical activity cancer prevention guidelines and aggregate the effects on cancer outcomes reported by the existing scientific literature. As such, the authors note its strengths and weaknesses, which are worth quoting at length:
Strengths of this systematic review include strict inclusion criteria to include only prospective studies that constructed adherence scores to the established cancer prevention guidelines by ACS or WCRF/AICR. All of the studies contained sizeable cohorts with multiple years of follow-up leading to sufficient sample sizes, ample power to detect associations, and sufficient number of outcomes, enabling them to evaluate associations for some site-specific cancers. However, there are also some limitations that must be considered. First, all studies generated their own adherence scores on the basis of recommendations from either the ACS or WCRF/AICR. Most studies assigned points for meeting or partially meeting recommendations, whereas others categorized adherence as “met” or “did not meet” recommendations. Including multiple levels of exposure may better capture the degree of adherence to the guidelines. Although ACS and WCRF/AICR guidelines are very similar, interpretations of how to measure the recommendations varied. Notably, physical activity was assessed several ways including in metabolic equivalents, times per week, and even a physical activity index. Furthermore, studies utilized frequency questionnaires to capture diet and physical activity data. These self-reported measures are well-known sources of measurement error, which may bias findings toward the null, lending to conservative findings in this review. Components of the adherence score were measured singularly at baseline and used to assess cancer risk over time. Repeated measurements of diet and physical activity may have provided an improved exposure assessment of long-term behavior and risk over time. Follow-up times ranged from 7.7 to 14 years, which may not be sufficient for assessing the protective role of adherence to nutrition and physical activity cancer prevention guidelines. In addition, although the studies evaluated large cohorts, there was limited population heterogeneity with regard to race or ethnicity, with the exception of the WHI and SCCS studies. Furthermore, analyses varied somewhat among the studies. All studies evaluating associations with ACS guideline adherence made comparisons of high versus low adherence. One study used WCRF/AICR guidelines to compare “met” versus “did not meet” recommendations (29), whereas a single study evaluated adherence to WCRF/AICR guidelines on the basis of point increments of the overall score (36). Finally, the potential for publication bias is always of concern. Studies with significant findings are more likely to be published than those with null or unimportant findings. Grey literature was included in the search via Google Scholar in an attempt to capture any work that hasn’t been formally published (abstracts, conference proceedings, etc.). Even though the studies differed in some measurements of individual score components, construction of the adherence score, specifics of the set of guidelines used, and analytic methods, it is important to note that studies generally demonstrated agreement in their findings even across countries with varying diet and physical activity patterns.
You get the idea. The studies chosen were generally of high quality, but their methodologies for assessing adherence to the ACS and WCRF/AICR guidelines varied considerably. In actuality, to me the very fact that studies of such disparate groups using differing methods of assessment are all finding more or less the same thing tells me that the assessment by Kohler et al is likely pretty robust.
Another thing that has to be considered. This is a systematic review of a very specific question: Does adherence to the ACS and/or WCRF/AICR guidelines for diet and exercise (which, when you come down to it, are strongly similar) decrease the risk of being diagnosed with cancer and ultimately dying of cancer? The answer is yes, and we know at least some of the cancers affected: breast, colorectal, and endometrial cancers. We also know cancers for which adherence to these guidelines probably has little effect: lung, prostate, and ovarian cancers. Of course, this is a very limited number of the cancers that afflict human beings. It’s quite likely that diet and exercise impact the risk of many other cancers. However, this review doesn’t really address the effects of specific dietary and exercise interventions. It doesn’t, for instance, tell us if this food or that food, this nutrient or that nutrient, is associated with increased or decreased cancer risk. Those are different questions, and they are actually questions better suited to RCTs than the question that Kohler et al examined.
The reason proponents of integrative medicine claim so many non-surgical, non-pharmacological interventions is because, although inflated claims are often made for them, they can actually work, in contrast to the pseudoscientific treatments also “integrated” into medicine, such as acupuncture, traditional Chinese medicine (TCM), and the like. There’s a reason why we at SBM have at various times referred to diet, exercise, and other science-based lifestyle interventions co-opted by integrative medicine as a “Trojan horse,” particularly in academia. Once academia brings the nice, shiny “horse” of “non-pharmacological interventions” into its citadel, out jump the Greeks of quackery.None of this, however, means that diet and exercise aren’t powerful tools to promote health. Rather, it’s that they are and should remain part of science-based medicine, rather than being tainted by association with the quackery of integrative medicine. And there is a lot of science out there to support the important role of diet as a modulator of cancer risk.
Unfortunately, taking all the evidence together for individual foods, nutrients, exercise, and diet becomes devilishly complicated and contradictory, too. For example, do you remember John Ioannidis’ systematic review of existing scientific evidence associating specific foods with cancer? I do, as Steve Novella and I both blogged about it. Basically, Ioannidis examined 50 common ingredients at random out of cookbooks. He then scoured the literature looking for studies showing an association (positive or negative) with cancer and found that 80% of the ingredients had such studies, often with studies linking them to cancer and others showing a protective effect. As I said at the time, that’s why it’s important to aggregate data. Whenever it comes to studies of diet and virtually any health outcome, there is always a lot of noise.
There is a lot of noise in Kohler’s study, too, but the results are consistent. You can reduce your risk of cancer by staying active and exercising, eating a healthy diet with a lot of plant-based foods and minimizing intake of processed meats, limiting alcohol consumption (although I think the WCRF/AICR guidelines go a bit too far in saying that you shouldn’t drink at all if possible), and maintaining a healthy weight. (Of course, if you stay active and eat a healthy diet, maintaining a healthy weight will probably not be a problem.) Conceptually, it’s easy to do. In practice, as I’m discovering, it’s anything but easy.